Overview
ABSTRACT
The direct current electrolysis of aqueous sodium chloride solutions allows for obtaining chlorine, soda and hydrogen. The basic principle of electrolysis cells is to maintain the two gases - chlorine and hydrogen- separated for safety reasons as well as the two products - chorine and soda - which may recombine. Three electrolysis processes are currently used: the "mercury" process, the "diaphragm" process and the "membrane" process.
Read this article from a comprehensive knowledge base, updated and supplemented with articles reviewed by scientific committees.
Read the articleAUTHOR
-
Jean-Christophe MILLET: Process Manager, Chlorochemicals Division - Arkema
INTRODUCTION
The direct current electrolysis of aqueous sodium chloride solutions produces chlorine, soda, and hydrogen. The overall chemical reaction is as follows:
involving two electrons.
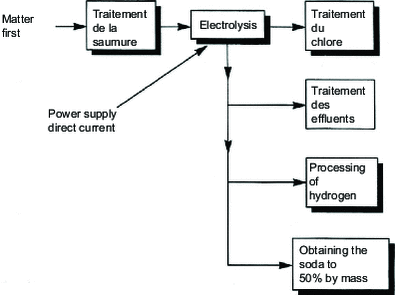
The general principle of the process requires five main sections:
brine purification treatment;
the electrolysis room;
chlorine treatment;
obtaining soda ash at 50% by mass;
hydrogen processing;
and can be represented by the schematic diagram in Figure 1 .
The basic principle of electrolysis cells is to keep the two gases (chlorine and hydrogen) separate for safety reasons (violent reaction between the two gases) and the two products (chlorine and soda) that are likely to recombine.
There are three different electrolysis processes currently in use:
the first with a mercury cathode, known as the "mercury" process;
the second with a percolating diaphragm separating the anode and cathode chambers, known as the "diaphragm" process;
the third with an ion exchange membrane as a separator between the anode and cathode chambers, known as the "membrane" process.
Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference
EDITIONS
Other editions of this article are available:
This article is included in
Unit operations. Chemical reaction engineering
This offer includes:
Knowledge Base
Updated and enriched with articles validated by our scientific committees
Services
A set of exclusive tools to complement the resources
Practical Path
Operational and didactic, to guarantee the acquisition of transversal skills
Doc & Quiz
Interactive articles with quizzes, for constructive reading
Chlorine (Cl2)
Bibliographic references
Organizations
Eurochlor http://www.eurochlor.org
Websites
Suppliers
Chlorine and soda
Arkema http://www.arkema.com
Solvay http://www.solvay.fr
Rhodia http://chloralp.com/fr/qsn_pres.html
SPC Harbonnières...
Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference