11. Main causes of uncertainty in equilibrium diagrams
11.1 Imprecision in certain areas
In many diagrams, the limits of certain domains are indicated by dotted lines, meaning that they are not known with sufficient precision. This is mainly the case for high and low temperatures. In the case of high temperatures, this inaccuracy is mainly due to pollution of the alloy either by the atmosphere used, or by the materials of the crucibles containing the alloys, mainly in the case of highly reactive refractory metal alloys (Cr, Ti, etc.), but also to difficulties in measuring high temperatures, and solidification conditions. At low temperatures, the main problem is the slowness of certain solid-state transformations, and even more so of solid-state diffusion. For some regions difficult to determine in certain diagrams, the limits of the domains...
Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference
This article is included in
Studies and properties of metals
This offer includes:
Knowledge Base
Updated and enriched with articles validated by our scientific committees
Services
A set of exclusive tools to complement the resources
Practical Path
Operational and didactic, to guarantee the acquisition of transversal skills
Doc & Quiz
Interactive articles with quizzes, for constructive reading
Main causes of uncertainty in equilibrium diagrams
Diagram classification
In this folder, the diagrams are arranged in alphabetical order of the symbol of the element concerned, which comes first in alphabetical order among the elements making up the alloy; then, for a given element, in alphabetical order of the symbol of the second element.
Example
we find Fe-C to C-Fe.
...
Binary alloys containing Ag

Binary alloys containing Al
Binary alloys containing As
As-Cu (arsenic-copper – figure )
This diagram has recently been completely revised. The relationships between phases and their compositions are now well known; there are apparently no other intermediate phases richer in arsenic than those shown in the diagram.
-
Phase structures
(Cu) : c. f. c....
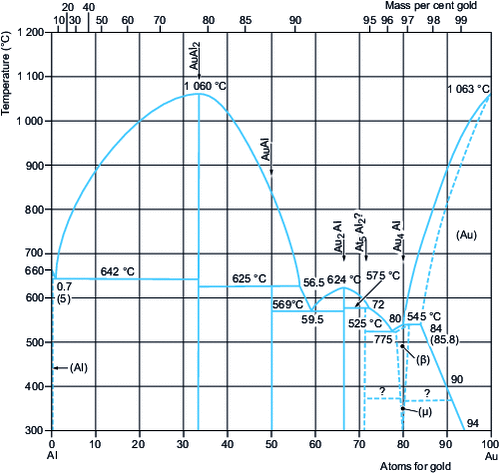
Au-containing binary alloys

Binary alloys containing either B, Be or Bi

Binary alloys containing C

Binary alloys containing either Ca, Cd or Ce

Binary alloys containing Co

Binary alloys containing Cr

Binary alloys containing Cu

Binary alloys containing Fe

Binary alloys containing Li, Mg or Mn

Binary alloys containing either Mo, or N, or Nb, or Ni, or O

Binary alloys containing either Pb, Sb, Sn or Ti

Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference