7. Solidification and segregation coefficient
During cooling, liquid solidification begins at the temperature of intersection of the vertical line drawn on the alloy composition x 0 diagram with the liquidus line (figure 18 ). When only a single solid phase is formed, the composition of the first crystals can be read on the diagram at the intersection of the horizontal isotherm at the start of solidification with the solidus line. As solidification continues, the temperature falls, the composition of the liquid follows the liquidus and that of the solid the solidus. In the case of figure 18 both phases in equilibrium become simultaneously enriched in element B, but through...
Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference
This article is included in
Studies and properties of metals
This offer includes:
Knowledge Base
Updated and enriched with articles validated by our scientific committees
Services
A set of exclusive tools to complement the resources
Practical Path
Operational and didactic, to guarantee the acquisition of transversal skills
Doc & Quiz
Interactive articles with quizzes, for constructive reading
Solidification and segregation coefficient
Diagram classification
In this folder, the diagrams are arranged in alphabetical order of the symbol of the element concerned, which comes first in alphabetical order among the elements making up the alloy; then, for a given element, in alphabetical order of the symbol of the second element.
Example
we find Fe-C to C-Fe.
...
Binary alloys containing Ag

Binary alloys containing Al
Binary alloys containing As
As-Cu (arsenic-copper – figure )
This diagram has recently been completely revised. The relationships between phases and their compositions are now well known; there are apparently no other intermediate phases richer in arsenic than those shown in the diagram.
-
Phase structures
(Cu) : c. f. c....
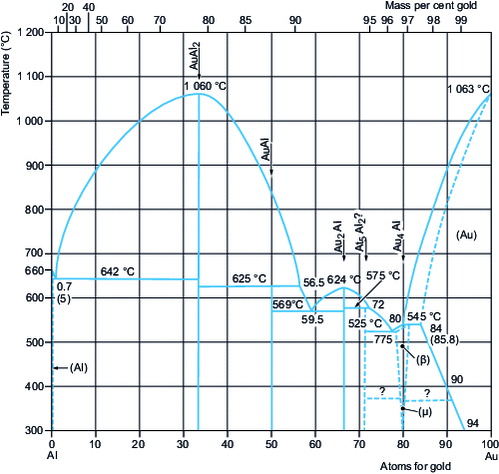
Au-containing binary alloys

Binary alloys containing either B, Be or Bi

Binary alloys containing C

Binary alloys containing either Ca, Cd or Ce

Binary alloys containing Co

Binary alloys containing Cr

Binary alloys containing Cu

Binary alloys containing Fe

Binary alloys containing Li, Mg or Mn

Binary alloys containing either Mo, or N, or Nb, or Ni, or O

Binary alloys containing either Pb, Sb, Sn or Ti

Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference