Overview
Read this article from a comprehensive knowledge base, updated and supplemented with articles reviewed by scientific committees.
Read the articleAUTHOR
-
Jean HERTZ: Professor Emeritus, Université Henri-Poincaré - Nancy I
INTRODUCTION
An alloy can exist in different states: as a crystallized solid, as an aggregate of microcrystals called grains, often of distinct crystalline varieties, or as a liquid or gas.
These different states represent the various phases contained in the alloy, which can change according to the conditions imposed on this "thermodynamic system", mainly chemical composition and temperature, but also pressure. For fixed conditions, a certain holding time at high temperature is required to stabilize the alloy system in an apparently definitive state, which is identified with the system's stable thermodynamic equilibrium. But there are also false equilibria, known as metastable equilibria. To achieve equilibrium, all constituent atoms must be able to move within all phases: this is known as chemical diffusion. In liquids and gases, diffusion is generally active and rapid, but in crystallized phases, a certain "diffusion temperature" must be exceeded to achieve internal atomic movement, which takes place by permutation with vacant sites on the crystal lattice. If a subterfuge (ultra-rapid quenching) succeeds in blocking atomic diffusion in a liquid and cooling it to room temperature without crystallization, the result is an amorphous glass or solid.
This is the experiment that enables us to observe which phases coexist in an alloy, as a function of chemical composition, equilibrium temperature and even applied pressure. It also enables us to determine the phase transition lines on which certain phases appear or disappear. In practice, it can be seen that the pressure variable has very little impact on the equilibrium of condensed liquid or solid phases, whether this state is single-phase or polyphase. The same is not true for volatile metals generating a gaseous phase. To observe the influence of the pressure variable on condensed phases, very high pressures, of the order of several kilobars, are required. For this reason, phase equilibria are often described assuming a fixed pressure, and using as variables the contents of the constituents and the temperature. For example, for a binary alloy, the abscissa shows the average concentration of one of the two constituents, while the ordinate shows the temperature. Such a representation is called a binary phase equilibrium diagram. With volatile metals, another convention is used: the natural vapor pressure is maintained in a constant volume; in this case, the phase diagram is not isobaric.
The very existence of equilibrium diagrams derives from the general laws of chemical thermodynamics. Since the advent of automatic calculation tools, chemical thermodynamics has made it possible to model phase diagrams numerically on the basis of its general laws, and thus to predict or confirm which phases coexist in the...
Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference
This article is included in
Studies and properties of metals
This offer includes:
Knowledge Base
Updated and enriched with articles validated by our scientific committees
Services
A set of exclusive tools to complement the resources
Practical Path
Operational and didactic, to guarantee the acquisition of transversal skills
Doc & Quiz
Interactive articles with quizzes, for constructive reading
Equilibrium diagrams
Diagram classification
In this folder, the diagrams are arranged in alphabetical order of the symbol of the element concerned, which comes first in alphabetical order among the elements making up the alloy; then, for a given element, in alphabetical order of the symbol of the second element.
Example
we find Fe-C to C-Fe.
...
Binary alloys containing Ag

Binary alloys containing Al
Binary alloys containing As
As-Cu (arsenic-copper – figure )
This diagram has recently been completely revised. The relationships between phases and their compositions are now well known; there are apparently no other intermediate phases richer in arsenic than those shown in the diagram.
-
Phase structures
(Cu) : c. f. c....
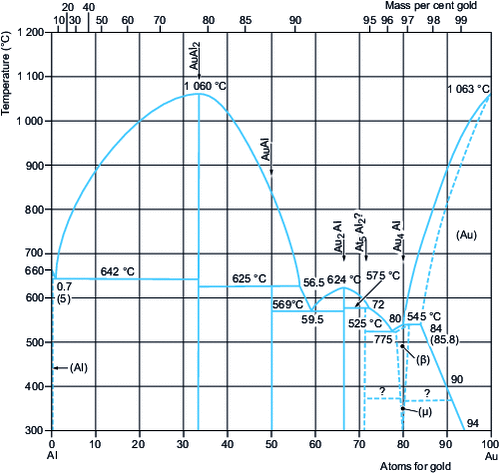
Au-containing binary alloys

Binary alloys containing either B, Be or Bi

Binary alloys containing C

Binary alloys containing either Ca, Cd or Ce

Binary alloys containing Co

Binary alloys containing Cr

Binary alloys containing Cu

Binary alloys containing Fe

Binary alloys containing Li, Mg or Mn

Binary alloys containing either Mo, or N, or Nb, or Ni, or O

Binary alloys containing either Pb, Sb, Sn or Ti

Exclusive to subscribers. 97% yet to be discovered!
You do not have access to this resource.
Click here to request your free trial access!
Already subscribed? Log in!

The Ultimate Scientific and Technical Reference